Molecular Weight
Molecular weight : 44.01 g/mol
Solid phase
Latent heat of fusion (1,013 bar, at triple point) : 196.104 kJ/kg
Solid density : 1562 kg/m3
Liquid phase
Liquid density (at -20 °C (or -4 °F) and 19.7 bar) : 1032 kg/m3
Liquid/gas equivalent (1.013 bar and 15 °C (per kg of solid)) : 845 vol/vol
Boiling point (Sublimation) : -78.5 °C
Latent heat of vaporization (1.013 bar at boiling point) : 571.08 kJ/kg
Vapor pressure (at 20 °C or 68 °F) : 58.5 bar
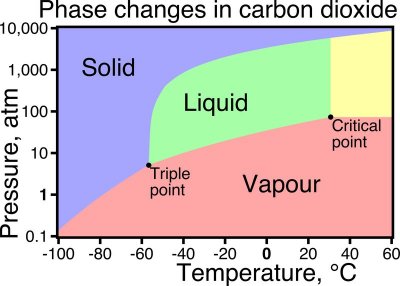
Critical point
Critical temperature : 31 °C
Critical pressure : 73.825 bar
Critical density : 464 kg/m3
 |
 |
 |
Triple point
Triple point temperature : -56.6 °C
Triple point pressure : 5.185 bar
Gaseous phase
Gas density (1.013 bar at sublimation point) : 2.814 kg/m3
Gas density (1.013 bar and 15 °C (59 °F)) : 1.87 kg/m3
Compressibility Factor (Z) (1.013 bar and 15 °C (59 °F)) : 0.9942
Specific gravity (air = 1) (1.013 bar and 21 °C (70 °F)) : 1.521
Specific volume (1.013 bar and 21 °C (70 °F)) : 0.547 m3/kg
Heat capacity at constant pressure (Cp) (1.013 bar and 25 °C (77 °F)) : 0.037 kJ/(mol.K)
Heat capacity at constant volume (Cv) (1.013 bar and 25 °C (77 °F)) : 0.028 kJ/(mol.K)
Ratio of specific heats (Gamma:Cp/Cv) (1.013 bar and 25 °C (77 °F)) : 1.293759
Viscosity (1.013 bar and 0 °C (32 °F)) : 0.0001372 Poise
Thermal conductivity (1.013 bar and 0 °C (32 °F)) : 14.65 mW/(m.K)
Miscellaneous
Solubility in water (1.013 bar and 0 °C (32 °F)) : 1.7163 vol/vol
Concentration in air : 0.03 vol %

